The ideal microbial colonization takes place when an infant undergoes vaginal birth and is exclusively breast-fed for the first 4-6 months, setting the stage for a healthy immune and metabolic foundation. As the infant grows, this microbiota evolves, resulting in a unique gut ecosystem by the age of three, which persists throughout life.
However, modern lifestyles have raised concerns. Enhanced sanitation, dietary shifts, immunizations, and antibiotic overuse in developed nations have altered our gut's bacterial makeup. This transformation may correlate with the increased prevalence of allergies, autoimmune diseases, and other immune disorders, highlighting the importance of understanding and maintaining the balance in our microbial world.
What is Dysbiosis?
Dysbiosis refers to an imbalance in our gut's microbiota. It is a change from the usual, beneficial microbial communities found in healthy individuals to a state that might contribute to diseases. While the term "dysbiosis" might appear vague, it essentially signifies any disruption to the typically beneficial bacteria within our digestive tracts. A pivotal question in the study of dysbiosis is whether this microbial imbalance is the cause or a result of the disease in question. The nuances of dysbiosis can be classified into the following different types:
1. Loss of Beneficial Microbes:
One manifestation of dysbiosis is the loss of known beneficial bacteria. Some specific bacteria, such as Bacteroides fragilis or certain Clostridium strains, play a critical role in the development and sustenance of T-regulatory cells (Tregs). Tregs prevent undue inflammatory responses to ordinary bacteria and self-antigens, acting as a protective shield against autoimmune diseases. Other bacteria, like Lactobacillus acidophilus or Bifidobacterium breve, have direct anti-inflammatory effects. An absence or reduction of these beneficial bacteria can disrupt the delicate balance our gut maintains between defending against pathogens and tolerating beneficial, commensal microbes.
2. Expansion of Harmful Microbes:
Another aspect of dysbiosis is the growth of harmful or potentially harmful bacteria, often termed as pathobionts. An increase in certain bacteria like Escherichia coli, Shigella, and Klebsiella, belonging to the Enterobacteriaceae family, can result in this type of dysbiosis. Genetic mutations affecting immune responses can also pave the way for an upsurge in these pathobionts, potentially leading to conditions like colitis. Specific genetic defects, for instance in receptors like TLR-5, can spur the growth of such pathobionts.
3. Diminished Diversity and Implications:
Finally, a significant indicator of dysbiosis is the loss of microbial diversity within the gut. A diverse bacterial community offers robust protection against many noncommunicable diseases (NCDs). For instance, a group of Clostridium species can stimulate Tregs more effectively than a single species. A decrease in this diversity can expose the host to potential health risks. With advancements in metagenomic analysis, it's now understood that many bacteria in the gut cannot be identified solely through traditional culture methods. The healthy human gut is mostly home to two major bacterial phyla—Firmicutes and Bacteroides—with others present in smaller quantities. Changes in these populations, such as an increase in the Proteobacteria phyla, have been linked to inflammatory and metabolic diseases. As research advances, specific bacterial markers for NCDs could emerge, potentially leading to new preventive or therapeutic strategies.
What are the symptoms of dysbiosis?
Dysbiosis manifests in a multitude of ways, affecting various systems within the body. Commonly reported gastrointestinal symptoms include bloating, gas, diarrhea, constipation, and irritable bowel syndrome (IBS). Beyond the gut, dysbiosis can be linked to more systemic issues such as fatigue, recurrent yeast infections, urinary tract infections, joint pain, and skin problems like acne and eczema. Moreover, emerging research has unveiled connections between gut health and cognitive functions. Some studies suggest that dysbiosis might play a role in mood disorders like depression and anxiety due to the gut-brain connection.
Another significant symptom is the presence of frequent and recurrent infections, signaling that the immune system is compromised. The gut microbiome is deeply intertwined with our immune system's health. Dysbiosis can lead to reduced immune function, making the body more susceptible to infections.
It's important to note that the field of dysbiosis is rapidly evolving, with new research emerging frequently. While correlations between dysbiosis and various health issues have been observed, it's crucial to differentiate between correlation and causation. Not all associations mean a direct cause-and-effect relationship. Therefore, further research is essential to establish more definitive links and understand the broader implications of dysbiosis on human health.
Early detection and understanding of these symptoms are crucial. As dysbiosis can lead to or exacerbate chronic health conditions, it's imperative to seek medical advice if one experiences persistent or worsening symptoms. By diagnosing and addressing dysbiosis early on, many associated health issues can be managed or even prevented.Causes of Dysbiosis
The stability and health of our gut microbiota play a significant role in our overall well-being. Disruptions or imbalances, known as dysbiosis, can lead to various health issues. Several factors, from perinatal events to genetic and environmental factors, can contribute to dysbiosis.
What causes dysbiosis?
1. Perinatal Causes of Dysbiosis
The perinatal period is pivotal for the initial colonization of the infant's gut. Disruptions during this time can have health implications later in life. Key factors during this period that can impact microbial balance include:
Maternal Weight Gain: Significant weight gain during pregnancy might alter the maternal microbiota. This change can affect the newborn during birth, potentially increasing the child's risk of health issues like excessive weight gain later in life.
Premature Birth: Infants born prematurely might show signs of dysbiosis, which can be influenced by factors like intrauterine infections. Such imbalances can contribute to conditions like necrotizing enterocolitis.
Cesarean Section Birth: Babies delivered via C-section may miss out on exposure to certain beneficial microbes present in the mother's birth canal. This could be associated with a higher risk of asthma, obesity, and celiac disease later in life.
Antibiotic Use: The use of antibiotics in infancy, especially when not medically necessary, can disrupt the gut's microbial balance. Such disruptions might be linked to a higher risk of conditions like asthma in adolescence.
Lack of Breastfeeding: Feeding infants exclusively with breast milk in the early months is important for the proper colonization of the gut. Deviations from this can potentially increase the risk of allergies, autoimmune diseases, and excessive weight gain.
2. Genetic Causes
Certain genetic markers can make an individual more susceptible to dysbiosis. For instance, variations in genes like TLR-4 or IL-10 may influence normal intestinal colonization, possibly leading to conditions like inflammatory bowel disease. The interaction between the microbiota and these genetic factors can be complex.
3. Dietary Causes
Diet can influence the composition of our gut microbiota. Diets high in animal fats and proteins, for example, might be associated with changes in the gut environment. Conversely, diets rich in fiber and complex carbohydrates, like the Mediterranean diet, may support a healthier gut. Additionally, certain food additives could disrupt the gut microbiota.
4. Disease Causes
Certain diseases might influence the state of gut microbiota. For instance, some individuals diagnosed with inflammatory bowel diseases might have had previous intestinal infections. The relationship between disease and dysbiosis is intricate, suggesting that an altered gut environment can, in some cases, exacerbate the disease, especially if there's a genetic predisposition.
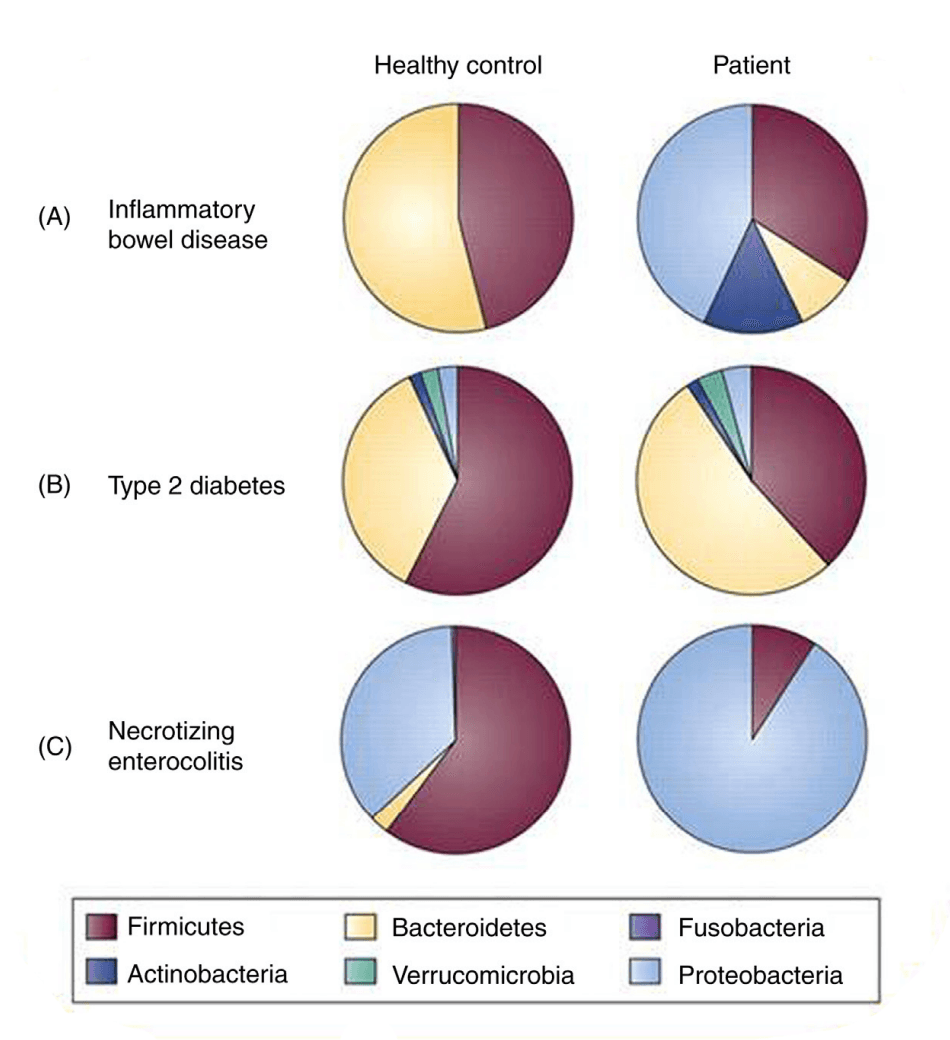
(Figure 1: Comparisons of bacterial phyla as determined by metaanalysis of patients with disease versus age-matched controls. Extracted from Walker, W.A., 2017)
5. Stress Causes
Preliminary evidence suggests there might be a connection between stress and gut health. Stress has been shown to impact gut microbiota composition, which in turn might influence certain neurological conditions. There are also early indications that probiotics could help address some neurological changes induced by stress. The potential influence of maternal stress during pregnancy on neonatal health is a topic of ongoing research.
In conclusion, understanding the factors leading to dysbiosis is important. Recognizing and addressing these factors can potentially help in preventing or managing several health conditions.
How is dysbiosis diagnosed?
1. Traditional Stool Analysis
This technique analyzes a fecal sample from the patient. Comprehensive stool tests assess the bacterial composition and can also identify pathogens, parasites, and yeasts that might be disrupting the balance. These tests usually provide quantitative measurements of beneficial bacteria, imbalanced bacteria, and bacteria that could be harmful.
2. Metagenomic Sequencing
This method analyzes a fecal sample to provide a comprehensive view of the gut microbiota. High-throughput sequencing allows for the detection and quantification of microbial genes present in the sample. By examining the DNA from the stool, metagenomic sequencing identifies both the types and functions of bacteria within the gut.
3. Organic Acid Test
This test analyzes a urine sample. Organic acids are the metabolic byproducts of body processes, and their presence in urine can provide insights into various metabolic dysfunctions. Some organic acids are produced by pathogenic bacteria, and their elevated levels in the urine can be indicative of dysbiosis.
What treatment options for dysbiosis are available?
Addressing dysbiosis requires a multi-faceted approach, combining dietary modifications, targeted interventions, and in some cases, medical treatments.
1. Dietary Changes
Diet plays a pivotal role in shaping the gut microbiota. A diet rich in fibers, whole grains, fruits, and vegetables supports the growth of beneficial bacteria in the gut. On the other hand, a high-fat, high-sugar "Western" diet can promote dysbiosis. Some patients benefit from tailored diets such as the low-FODMAP diet, which reduces fermentable sugars that can cause dysbiosis and symptoms in susceptible individuals.
2. Lifestyle Changes
Regular physical activity has been shown to enrich the diversity of gut microbiota. Exercise can increase the abundance of beneficial microbial species that produce short-chain fatty acids, like butyrate, which provides energy to gut cells and has anti-inflammatory effects.
Chronic stress can lead to alterations in the gut microbiota. Techniques like mindfulness, meditation, yoga, and deep-breathing exercises can help manage stress and potentially mitigate its negative impact on the gut microbiota. It's essential to understand that while stress can influence gut health, gut health can conversely affect emotional and mental well-being, emphasizing the importance of the gut-brain axis.
Lastly, sleep plays a vital role in maintaining a balanced gut microbiota. Chronic sleep deprivation can lead to dysbiosis. It's crucial to prioritize good sleep habits, maintain a consistent sleep schedule, and ensure 7-9 hours of sleep nightly for most adults.
3. Probiotics
These are live bacteria that confer health benefits to the host when administered in appropriate amounts. The consumption of probiotics can help restore the balance of gut bacteria. Lactobacillus and Bifidobacterium are two commonly used strains that have shown benefits in many studies.
4. Fecal Microbiota Transplantation (FMT)
For severe cases of dysbiosis, especially those caused by recurrent Clostridium difficile infections, FMT has emerged as a promising treatment. It involves transferring fecal bacteria from a healthy donor to the patient, helping to reset the gut's microbial environment.
Please note: While FMT is promising, it's a procedure that should be conducted under strict medical supervision and isn't suitable for all cases of dysbiosis.
Our understanding of the diverse microbes living in our intestines has grown substantially. We're making progress in pinpointing specific types of bacteria associated with particular diseases. This knowledge can help us identify disease markers and even guide treatments and prevention strategies. A significant discovery is the link between the lack of certain beneficial bacteria, like Lactobacillus or Bifidobacteria, and the development of allergic diseases. Providing these bacteria as probiotics during infancy has shown potential in preventing such allergies. Moreover, distinct bacteria profiles have been associated with obesity, and introducing these missing bacteria has exhibited some promising results in managing the condition. In extreme cases of imbalance, such as persistent Clostridium difficile diarrhea, fecal transplants might be necessary.
Throughout this exploration, we've highlighted the essential role of balanced gut bacteria, especially during infancy, for immune system development and overall health. Factors that disrupt this balance, leading to dysbiosis, have been scrutinized. As we move forward, more detailed research is essential to determine the specific bacterial profiles of diseases and explore new ways to address them.
Sources:
Walker, W.A. (2017). Dysbiosis. Harvard Medical School, Boston, MA, United States; Mucosal Immunology and Biology Research Center, Pediatric Gastroenterology and Nutrition, Massachusetts General Hospital for Children, Boston, MA, United States.
Hooper LV, Littman DR, Macpherson AJ. (2012). Interactions between the microbiota and the immune system. Science, 336, 1268–73.
Arpaia N, Campbell C, Fan X, et al. (2013). Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature, 504, 451–5.
Rodriquez JM, Murphy K, Stanton C, et al. (2015). The composition of the gut microbiota throughout life, with an emphasis on early life. Microb Ecol Health Dis, 26, 26050.
Aagaard K, Ma J, Antony KM, Ganu R, Peterosino J, Versalovic J. (2014). The placenta harbors a unique microbiome. Sci Transl Med, 8, e66986.
Houghteling P, Walker WA. (2015). Why is initial bacterial colonization of the intestine important to the infant’s and child’s health. J Pediatr Gastro Nutr, 60, 294–307.
Round JL, Mazmanian SK. (2009). The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol, 9, 313–23.
Clemente JC, Ursell LK, Parfrey LW, Knight R. (2012). The impact of the gut microbiota on human health: An integrative view. Cell, 148, 1258–70.
Wu HJ, Wu E. (2012). The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes, 3, 4–14.
Bisgaard H, Li N, Bonnelykke K, et al. (2011). Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol, 128, 646–52.
De Filippo C, Cavalieri D, Di Paola M, et al. (2010). Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. PNAS, 107, 14691–6.
Wang Z, Klipfell E, Bennett BJ, et al. (2011). Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature, 472, 57–63.
Sekirov, I., Russell, S. L., Antunes, L. C. M., & Finlay, B. B. (2010). Gut microbiota in health and disease. Physiological Reviews, 90(3), 859-904.
Foster, J. A., & Neufeld, K. A. M. (2013). Gut–brain axis: how the microbiome influences anxiety and depression. Trends in neurosciences, 36(5), 305-312.
Lynch, S. V., & Pedersen, O. (2016). The human intestinal microbiome in health and disease. New England Journal of Medicine, 375(24), 2369-2379.
Le Chatelier E, Nielsen T, Qin J, et al. (2013). Richness of human gut microbiome correlates with metabolic markers. Nature, 500, 541–6.
Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. (2005). Obesity alters gut microbial ecology. PNAS, 102, 11070–5.
Koenig JE, Spor A, Scalfone N, et al. (2011). Succession of microbial consortia in the developing infant gut microbiome. PNAS, 108, 4578–85.
David LA, Maurice CF, Carmody RN, et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature, 505, 559–63.
Atarashi K, Tanoue T, Oshima K, et al. (2013). Treg induction by a rationally selected mixture of Clostridia strains from the human microbiota. Nature, 500, 232–6.
Smith PM, Howitt MR, Panikov N, et al. (2013). The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science, 341, 569–73.
Tilg H, Moschen AR. (2014). Microbiota and diabetes: An evolving relationship. Gut, 63, 1513–21.
Kamada N, Seo SU, Chen GY, Núñez G. (2013). Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol, 13, 321–35.
Qin J, Li R, Raes J, et al. (2010). A human gut microbial gene catalogue established by metagenomic sequencing. Nature, 464, 59–65.
Frank DN, St. Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. (2007). Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. PNAS, 104, 13780–5.
Ivanov II, Atarashi K, Manel N, et al. (2009). Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell, 139, 485–98.
Lepage P, Häsler R, Spehlmann ME, et al. (2011). Twin study indicates loss of interaction between microbiota and mucosa of patients with ulcerative colitis. Gastroenterology, 141, 227–36.
Ridaura VK, Faith JJ, Rey FE, et al. (2013). Gut microbiota from twins discordant for obesity modulate metabolism in mice. Science, 341, 1241214.
Qin J, Li Y, Cai Z, et al. (2012). A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature, 490, 55–60.
Larsen N, Vogensen FK, van den Berg FW, et al. (2010). Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PloS ONE, 5, e9085.
Forslund K, Hildebrand F, Nielsen T, et al. (2015). Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature, 528, 262–6.
Morgan XC, Tickle TL, Sokol H, et al. (2012). Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol, 13, R79.
Dethlefsen L, Relman DA. (2011). Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. PNAS, 108, 4554–61.
Dethlefsen L, Huse S, Sogin ML, Relman DA. (2008). The pervasive effects of an antibiotic on the human gut microbiota, as revealed by deep 16S rRNA sequencing. PLoS Biol, 6, e280.
Cho I, Yamanishi S, Cox L, et al. (2012). Antibiotics in early life alter the murine colonic microbiome and adiposity. Nature, 488, 621–6.
Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. (2013). Infant antibiotic exposures and early-life body mass. Int J Obes, 37, 16–23.
Cox LM, Yamanishi S, Sohn J, et al. (2014). Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell, 158, 705–21.
Sonnenburg JL, Bäckhed F. (2016). Diet–microbiota interactions as moderators of human metabolism. Nature, 535, 56–64.
Zhernakova A, Kurilshikov A, Bonder MJ, et al. (2016). Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science, 352, 565–9.
Rooks MG, Garrett WS. (2016). Gut microbiota, metabolites and host immunity. Nat Rev Immunol, 16, 341–52.
Yatsunenko T, Rey FE, Manary MJ, et al. (2012). Human gut microbiome viewed across age and geography. Nature, 486, 222–7.
Leung MH, Wilkins D, Lee PK. (2015). Insights into the pan-microbiome: skin microbial communities of Chinese individuals differ from other racial groups. Sci Rep, 5, 11845.
Spor A, Koren O, Ley R. (2011). Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol, 9, 279–90.
Knights D, Lassen KG, Xavier RJ. (2013). Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut, 62, 1505–10.
Kadooka Y, Sato M, Imaisum K, et al. (2010). Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr, 64, 636–43.
Quig, D. (1996). CDSA Test: A Three-in-One Medical Test. Alternative Medicine Review, 1(1), 15-26.
Sharpton, T. J. (2014). An introduction to the analysis of shotgun metagenomic data. Frontiers in plant science, 5, 209.
Lord, R. S., & Bralley, J. A. (2008). Clinical applications of urinary organic acids. Part 2. Dysbiosis markers. Alternative Medicine Review, 13(4), 292-306.
Hill, C., Guarner, F., Reid, G., Gibson, G. R., Merenstein, D. J., Pot, B., ... & Calder, P. C. (2014). Expert consensus document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nature Reviews Gastroenterology & Hepatology, 11(8), 506-514.
David, L. A., Maurice, C. F., Carmody, R. N., Gootenberg, D. B., Button, J. E., Wolfe, B. E., ... & Turnbaugh, P. J. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature, 505(7484), 559-563.
Kelly, C. R., Khoruts, A., Staley, C., Sadowsky, M. J., Abd, M., Alani, M., ... & Chen, L. A. (2016). Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: a randomized trial. Annals of internal medicine, 165(9), 609-616.
Staudacher H.M., Whelan K. (2017) The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut.
Allen J.M., Mailing L.J., Niemiro G.M., et al. (2018) Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Medicine & Science in Sports & Exercise.
Salleh M.R. (2008) Life event, stress and illness. The Malaysian Journal of Medical Sciences.
Carabotti M., Scirocco A., Maselli M.A., Severi C. (2015) The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Annals of Gastroenterology.
Benedict C., Vogel H., Jonas W., et al. (2016) Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Molecular Metabolism.
Your 14-Day Journey to Gut Health Begins Now!
Don't let digestive issues hold you back any longer—take the first step towards a healthier, happier you today